Science
Science
Science
LB-100 is the first PP2A-inhibitor with a promising safety profile
LB-100 is a small molecule inhibitor of the PP2A phosphatase, a critical enzyme involved in multiple cellular functions.
- Demonstrated safety in phase 1 clinical trials
- Demonstrated anti-cancer activity
in 25+ publications - Convenient IV delivery and cost-effective manufacturing
- Good Manufacturing Practice production in place
- FDA: Investigational New Drug status
- European Medical Agency: Investigational Medicinal Product Dossier approval Europe in 2022

LB-100 is the first PP2A-inhibitor with a promising safety profile
LB-100 is a small molecule inhibitor of the PP2A phosphatase, a critical enzyme involved in multiple cellular functions.
- Demonstrated safety in phase 1 clinical trials
- Demonstrated anti-cancer activity
in 25+ publications - Convenient IV delivery and cost-effective manufacturing
- Good Manufacturing Practice production in place
- FDA: Investigational New Drug status
- European Medical Agency: Investigational Medicinal Product Dossier approval Europe in 2022

LB-100 is the first PP2A-inhibitor with a promising safety profile
LB-100 is a small molecule inhibitor of the PP2A phosphatase, a critical enzyme involved in multiple cellular functions.
- Demonstrated safety in phase 1 clinical trials
- Demonstrated anti-cancer activity
in 25+ publications - Convenient IV delivery and cost-effective manufacturing
- Good Manufacturing Practice production in place
- FDA: Investigational New Drug status
- European Medical Agency: Investigational Medicinal Product Dossier approval Europe in 2022
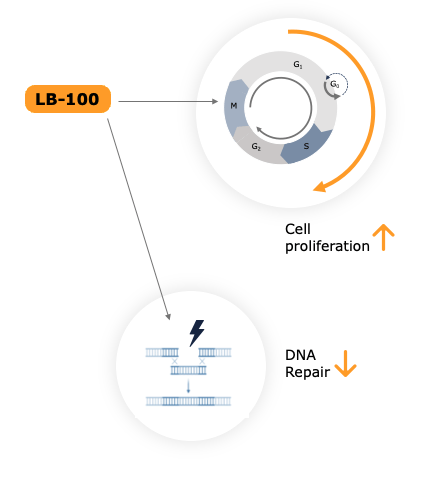
Chemotherapy: Mechanism of action of our PP2A inhibitor
LB-100 stimulates cell proliferation and inhibits DNA repair
This renders cancer cells more susceptible to chemotherapy agents, thus enhancing the treatment’s effectiveness.
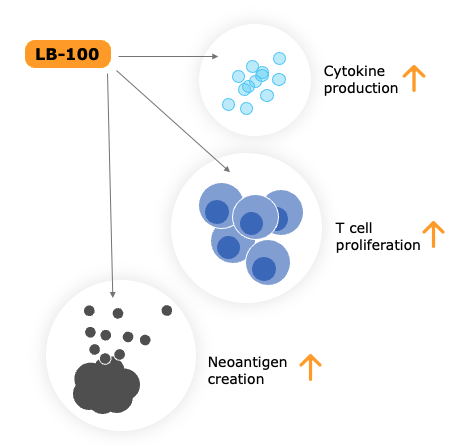
Immunotherapy: Mechanism of action of our PP2A inhibitor
LB-100 promotes the production of neoantigens and cytokines and enhances T cell proliferation.
This reinforces the systemic immune response to cancer, thus enhancing the effectiveness of immunotherapy.
We already have proof of concept that it works.
Inhibition of PP2A by LB-100 is effective in numerous cancer models
LB-100 has been shown to inhibit a spectrum of human cancers (1)
In extensive preclinical studies, LB-100, when coupled with standard cytotoxic drug therapies, potentiates the effectiveness of such regimens against hematologic cancers and solid tumors without enhancing toxicity.
In addition, as recently shown, low doses of LB-100 significantly increase the effectiveness of PD-1 immune-checkpoint blockade by activating cytotoxic T cells and CAR-T cells.
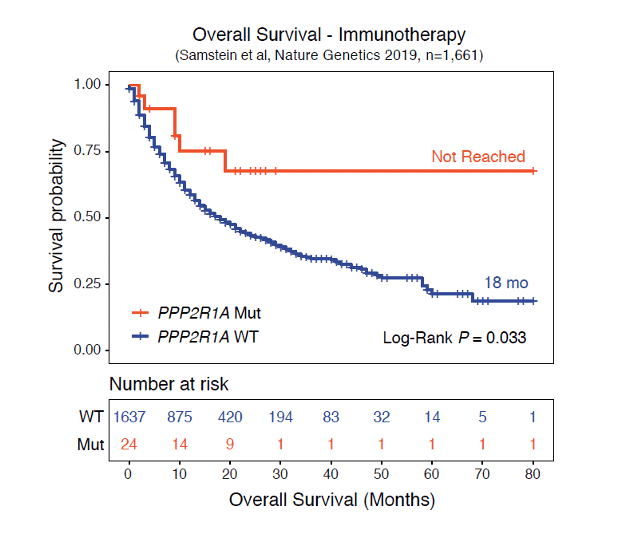
The 2019 study by Samstein published in Nature Genectics (Fig. 1) shows that tumors with a PP2A inactivating mutation (in the red line) have a much better response and thus survival when treated with immunotherapy. Lixte is developing a similar concept by inhibiting PP2A which could lead to the same improved response to immunotherapy.
Expanding Horizons in Oncology
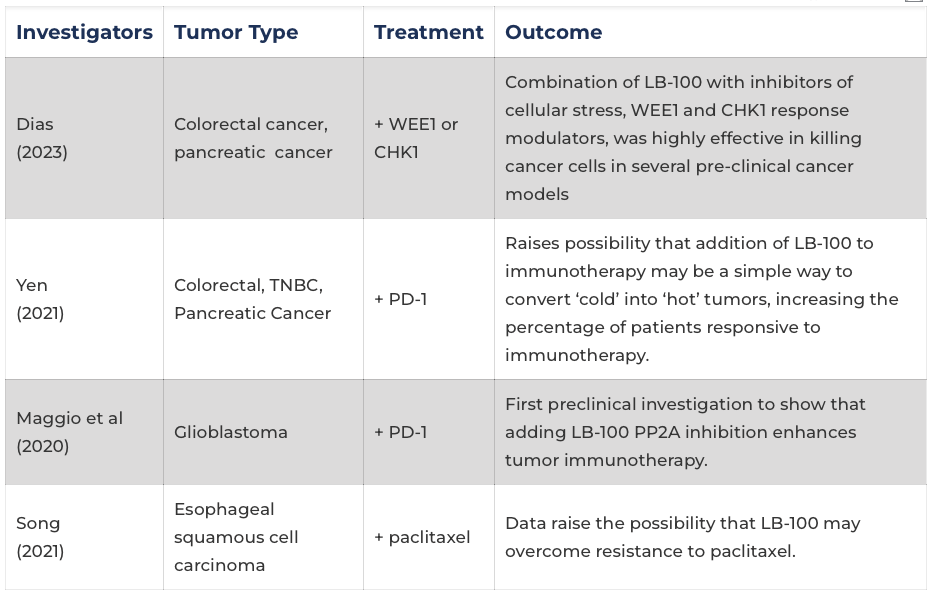
Key Preclinical Studies of LB-100
Investigators |
Tumor Type |
Treatment |
Outcome |
| Dias (2023) |
Colorectal cancer, pancreatic cancer | + WEE1 or CHK1 | Combination of LB-100 with inhibitors of cellular stress, WEE1 and CHK1 response modulators, was highly effective in killing cancer cells in several pre-clinical cancer models |
| Yen (2021) |
Colorectal, TNBC, Pancreatic Cancer | + PD-1 | Raises possibility that addition of LB-100 to immunotherapy may be a simple way to convert ‘cold’ into ‘hot’ tumors, increasing the percentage of patients responsive to immunotherapy. |
| Maggio et al (2020) |
Glioblastoma | + PD-1 | First preclinical investigation to show that adding LB-100 PP2A inhibition enhances tumor immunotherapy. |
| Song (2021) |
Esophageal squamous cell carcinoma | + paclitaxel | Data raise the possibility that LB-100 may overcome resistance to paclitaxel. |
Scientific References
Recently published articles supporting Lixte’s clinical development plan.
Safety
- Chung V et. al. Safety, Tolerability, and Preliminary Activity of LB-100, an Inhibitor of Protein Phosphatase 2A, in Patients with Relapsed Solid Tumors: An Open-Label, Dose Escalation, First-in-Human, Phase I Trial. Clin Cancer Res. 2017;23(13):3277-84.
Immunotherapy combinations
- Ho WS et. al. Pharmacologic inhibition of protein phosphatase-2A achieves durable immune-mediated antitumor activity when combined with PD-1 blockade. Nat Commun. 2018;9(1):2126.
- Yen YT et. al. Protein phosphatase 2A inactivation induces microsatellite instability, neoantigen production and immune response. Nat Commun. 2021;12(1):7297.
- Ho WS et. al. PP2Ac/STRN4 negatively regulates STING-type I IFN signaling in tumor-associated macrophages. The Journal of Clinical Investigation. 2023;133(6).
- Maggio D et. al. Inhibition of protein phosphatase-2A with LB-100 enhances antitumor immunity against glioblastoma. J Neurooncol. 2020;148(2):231-44.
- Samstein RM et. al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-6.
- Mirzapoiazova T et al. Protein Phosphatase 2A as a Therapeutic Target in Small Cell Lung Cancer. Mol Cancer Ther. 2021 Oct;20(10):1820-1835. doi: 10.1158/1535-7163.MCT-21-0013. Epub 2021 Jul 12.PMID: 34253596
- Hinchcliff E et al. Loss-of-function mutations in PPP2R1A Correlate with Exceptional Survival in Ovarian Clear Cell Carcinomas Treated with Immune Checkpoint Inhibitors. Gynecologic Oncology Volume 166, Supplement 1, August 2022, Page S66. doi: 10.1016/S0090-8258(22)01325-7
Chemotherapy combinations
- Song Q et. al. Pharmacological Inhibition of PP2A Overcomes Nab-Paclitaxel Resistance by Downregulating MCL1 in Esophageal Squamous Cell Carcinoma (ESCC). Cancers (Basel). 2021;13(19).
- Chang KE et. al. The protein phosphatase 2A inhibitor LB100 sensitizes ovarian carcinoma cells to cisplatin-mediated cytotoxicity. Mol Cancer Ther. 2015;14(1):90-100.
- Hong CS et. al. LB100, a small molecule inhibitor of PP2A with potent chemo- and radio-sensitizing potential. Cancer Biol Ther. 2015;16(6):821-33.
Future clinical development
- Dias MH et. al. Paradoxical activation of oncogenic signaling as a cancer treatment strategy. bioRxiv. 2023:2023.02.06.527335.
