Transforming cancer treatment with Protein Phosphatase 2A inhibitors
Unveiling the first of a novel class of therapeutic agents
Transforming cancer treatment with Protein Phosphatase 2A inhibitors
Unveiling the first of a novel class of therapeutic agents
Transforming cancer treatment with Protein Phosphatase 2A inhibitors
Unveiling the first of a novel class of therapeutic agents
Lixte has developed a first-in-class enhancer of chemotherapy and immunotherapy
Lixte Biotechnology Holdings, Inc. (Nasdaq: LIXT) is a clinical-stage pharmaceutical company developing a new class of oncology treatments and cancer therapies called PP2A inhibitors.
In recent years, the field of cancer treatment has undergone remarkable advancement with the introduction of immunotherapy. This approach has revolutionized the landscape of cancer care.
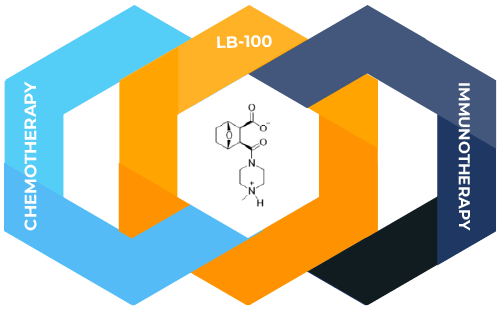
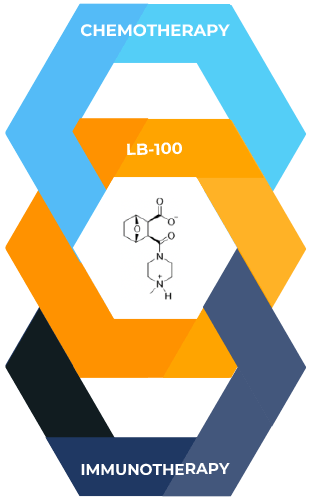
Both chemotherapy, and immunotherapy are treatment modalities that offer broad applicability to many types and stages of cancer, with the potential to benefit a wide range of patients. Our lead molecule LB-100 has shown remarkable synergy with chemotherapy and immunotherapy in a range of pre-clinical models.
Lixte has developed a first-in-class enhancer of chemotherapy and immunotherapy
Lixte Biotechnology Holdings, Inc. (Nasdaq: LIXT) is a clinical-stage pharmaceutical company developing a new class of oncology treatments and cancer therapies called PP2A inhibitors.
In recent years, the field of cancer treatment has undergone remarkable advancement with the introduction of immunotherapy. This approach has revolutionized the landscape of cancer care.
Both chemotherapy, and immunotherapy are treatment modalities that offer broad applicability to many types and stages of cancer, with the potential to benefit a wide range of patients. Our lead molecule LB-100 has shown remarkable synergy with chemotherapy and immunotherapy in a range of pre-clinical models.
Lixte has developed a first-in-class enhancer of chemotherapy and immunotherapy
Lixte Biotechnology Holdings, Inc. (Nasdaq: LIXT) is a clinical-stage pharmaceutical company developing a new class of oncology treatments and cancer therapies called PP2A inhibitors.
In recent years, the field of cancer treatment has undergone remarkable advancement with the introduction of immunotherapy. This approach has revolutionized the landscape of cancer care.
Both chemotherapy, and immunotherapy are treatment modalities that offer broad applicability to many types and stages of cancer, with the potential to benefit a wide range of patients. Our lead molecule LB-100 has shown remarkable synergy with chemotherapy and immunotherapy in a range of pre-clinical models.
Chemotherapy & Immunotherapy: Shared Potential, Common Challenges
Chemotherapy and immunotherapy face common challenges that have limited their success rates:
Limited efficacy: The efficacy of these approaches is limited by the resistance of cancer cells, both intrinsic and acquired.
Toxicity: The potential for side effects can restrict the dosage or duration of treatment and, consequently, impact the overall efficacy.
Pioneering a novel class of cancer therapy
CHEMOTHERAPY
+ LB-100
Enhanced chemotherapy efficacy
- Stimulates cell cycle
- Inhibits DNA repair

IMMUNOTHERAPY
+ LB-100
Enhanced immunotherapy efficacy
- Enhances T cell proliferation
- Increases release of cytokines
- Promotes production of neoantigens
Our unique approach: potentially enhances both immunotherapies and chemotherapies with a PP2A inhibitor
In addition to pursuing novel therapies or new combinations in oncology, we believe we should try to increase the efficacy of existing treatments so that they work better for a broader range of patients.
Our company has pioneered a novel class of cancer therapy that strongly enhances the efficacy of both chemotherapies and immunotherapies in pre-clinical models.
At the core of this approach lies our proprietary compound, LB-100, which acts as an inhibitor of the PP2A phosphatase—a critical enzyme involved in multiple cellular functions.
Latest Press Releases
FEBRUARY 18, 2026 8:00 AM EST
LIXTE Biotechnology Appoints Sidney Braun as CEO of its Liora Technologies Europe Ltd. Subsidiary
JANUARY 21, 2026 8:00 AM EST
DE LA SOUL-LED “GOOD HEALTH SUMMIT” LAUNCHES FEBRUARY 19 AT MOREHOUSE COLLEGE
JANUARY 20, 2026 8:00 AM EST
LIXTE Biotechnology Attending the DealFlow Discovery Conference, January 28-29



