Programs
Programs
Programs
Building a pipeline in multiple cancer indications
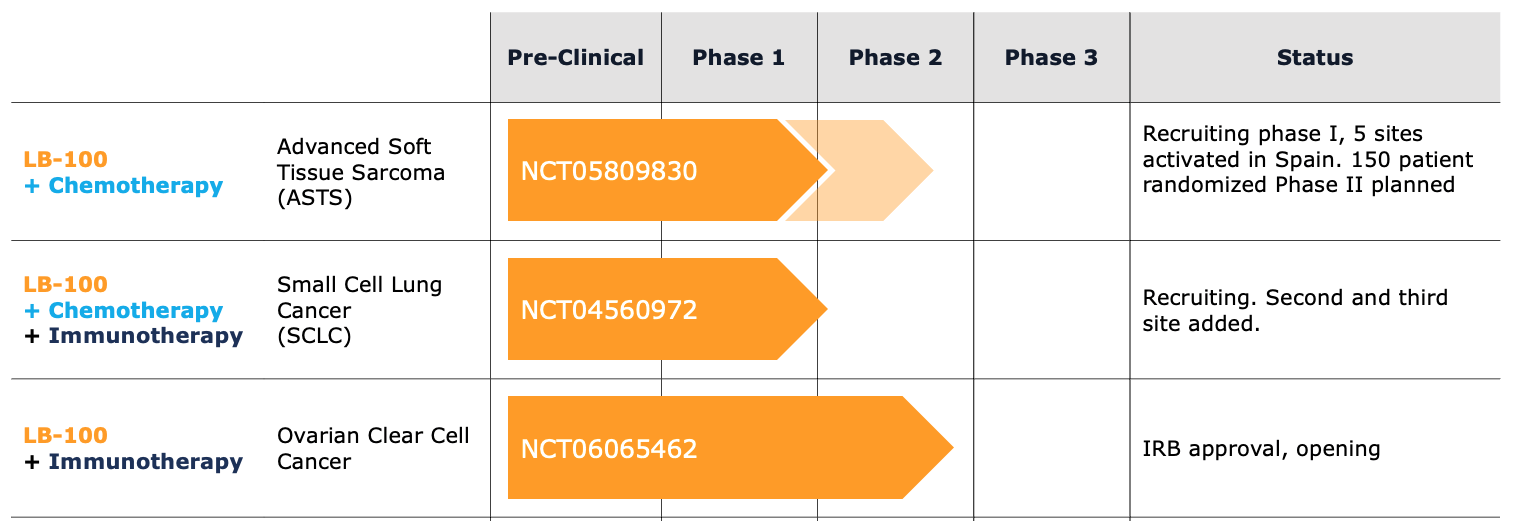
LIXTE is conducting multiple clinical trials with LB-100 in combination with chemotherapy and immunotherapy for solid tumors such as Advanced Soft Tissue Sarcoma, Advanced Small Cell Lung Cancer and Ovarian Clear Cell Cancer.
Building a pipeline in multiple cancer indications
LIXTE is conducting multiple clinical trials with LB-100 in combination with chemotherapy and immunotherapy for solid tumors such as Advanced Soft Tissue Sarcoma, Advanced Small Cell Lung Cancer and Ovarian Clear Cell Cancer.

Building a pipeline in multiple cancer indications
LIXTE is conducting multiple clinical trials with LB-100 in combination with chemotherapy and immunotherapy for solid tumors such as Advanced Soft Tissue Sarcoma, Advanced Small Cell Lung Cancer and Ovarian Clear Cell Cancer.

LB-100 + Chemotherapy
Advanced Soft Tissue Sarcoma
NCT05809830
Rationale
- ASTS is also a highly aggressive cancer with no effective treatment
- A single drug, doxorubicin, has been the standard Rx for over 40 years
- In animal models of ASTS, LB-100 markedly improves the effectiveness of doxorubicin
- Phase II, Only patients with ASTS subtypes that progress on average within 4.5 months of starting treatment are entered
Phase I: To define the safety of combining LB-100 with doxorubicin: one year of accrual.
Phase II: Untreated patients randomized to standard therapy with doxorubicin +/- LB-100 (maximum 150 patients) Readout: increased progression free survival from 4.5 to 7.5 months
Multi-center study in collaboration with Grupo Español de Investigación en Sarcomas (GEIS) (PI: Javier Martín Broto MD, Madrid) (12 sites in Spain)
Study open, first patient entered in June 2023
Rationale
- ASTS is also a highly aggressive cancer with no effective treatment
- A single drug, doxorubicin, has been the standard Rx for over 40 years
- In animal models of ASTS, LB-100 markedly improves the effectiveness of doxorubicin
- Phase II, Only patients with ASTS subtypes that progress on average within 4.5 months of starting treatment are entered
Study Design
Phase 2 Trial in
Advanced Soft Tissue Sarcoma (ASTS)
(NCT05809830)
Phase I: To define the safety of combining LB-100 with doxorubicin: one year of accrual.
Phase II: Untreated patients randomized to standard therapy with doxorubicin +/- LB-100 (maximum 150 patients) Readout: increased progression free survival from 4.5 to 7.5 months
Multi-center study in collaboration with Grupo Español de Investigación en Sarcomas (GEIS) (PI: Javier Martín Broto MD, Madrid) (12 sites in Spain)
Study open, first patient entered in June 2023
Rationale
- ASTS is also a highly aggressive cancer with no effective treatment
- A single drug, doxorubicin, has been the standard Rx for over 40 years
- In animal models of ASTS, LB-100 markedly improves the effectiveness of doxorubicin
- Phase II, Only patients with ASTS subtypes that progress on average within 4.5 months of starting treatment are entered
Study Design
Phase 2 Trial in
Advanced Soft Tissue Sarcoma (ASTS)
(NCT05809830)
Phase I: To define the safety of combining LB-100 with doxorubicin: one year of accrual.
Phase II: Untreated patients randomized to standard therapy with doxorubicin +/- LB-100 (maximum 150 patients) Readout: increased progression free survival from 4.5 to 7.5 months
Multi-center study in collaboration with Grupo Español de Investigación en Sarcomas (GEIS) (PI: Javier Martín Broto MD, Madrid) (12 sites in Spain)
Study open, first patient entered in June 2023
LB-100 + Chemotherapy
Advanced Small Cell Lung Cancer
NCT04560972
Rationale
- SCLC, the most aggressive form of lung cancer, has limited response to the current “best” therapy,
- Previously untreated extensive stage SCLC shows a median time to progression of 5.2 months and a median overall survival of 12.3 months.
- LB-100 exhibits activity as a standalone treatment in animal models of SCLC and increases efficacy of the standard regimen
Dr. Ravi Salgia, Chief of Medical Oncology and lung cancer expert at the City of Hope Cancer Center, is leading a Phase1b trial to test whether LB-100 improves standard therapy of previously untreated SCLC.
After the maximally tolerated dose of LB-100 is established when added to standard therapy, 12 patients will be treated at that dose to establish the recommended phase 2 dose (RP2D).
Multi-center study in collaboration with the City of Hope Cancer Center and the Sarah Cannon Research Institute.
Rationale
- SCLC, the most aggressive form of lung cancer, has limited response to the current “best” therapy,
- Previously untreated extensive stage SCLC shows a median time to progression of 5.2 months and a median overall survival of 12.3 months.
- LB-100 exhibits activity as a standalone treatment in animal models of SCLC and increases efficacy of the standard regimen
Study design
Phase 1b
Advanced Small Cell Lung Cancer (SCLC)
(NCT04560972)
Dr. Ravi Salgia, Chief of Medical Oncology and lung cancer expert at the City of Hope Cancer Center, is leading a Phase1b trial to test whether LB-100 improves standard therapy of previously untreated SCLC.
After the maximally tolerated dose of LB-100 is established when added to standard therapy, 12 patients will be treated at that dose to establish the recommended phase 2 dose (RP2D).
Multi-center study in collaboration with the City of Hope Cancer Center and the Sarah Cannon Research Institute.
Rationale
- SCLC, the most aggressive form of lung cancer, has limited response to the current “best” therapy,
- Previously untreated extensive stage SCLC shows a median time to progression of 5.2 months and a median overall survival of 12.3 months.
- LB-100 exhibits activity as a standalone treatment in animal models of SCLC and increases efficacy of the standard regimen
Study design
Phase 1b
Advanced Small Cell Lung Cancer (SCLC)
(NCT04560972)
Dr. Ravi Salgia, Chief of Medical Oncology and lung cancer expert at the City of Hope Cancer Center, is leading a Phase1b trial to test whether LB-100 improves standard therapy of previously untreated SCLC.
After the maximally tolerated dose of LB-100 is established when added to standard therapy, 12 patients will be treated at that dose to establish the recommended phase 2 dose (RP2D).
Multi-center study in collaboration with the City of Hope Cancer Center and the Sarah Cannon Research Institute.
LB-100 + Immunotherapy
Ovarian Clear Cell Cancer
NCT06065462
Rationale
- Ovarian Clear Cell Cancer (OCCC) with acquired mutations reducing PP2A activity are more sensitive to immunotherapy
- If LB-100 pharmacologic inhibition of PP2A mimics the effect of mutational reduction in PP2A, LB-100 is expected to enhance immunotherapy in OCCC tumors that do not have mutation
Study Design
Phase 2
Ovarian Clear Cell Cancer (OCCC)
(NCT06065462)
A Phase 2 collaborative clinical trial to assess whether adding Lixte’s LB-100 to GSK’s programmed death receptor-1 (PD-1)-blocking monoclonal antibody, dostarlimab, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (OCCC).
The clinical trial is sponsored by The University of Texas MD Anderson Cancer Center and will be conducted at MD Anderson and at Northwestern University’s Robert H. Lurie Comprehensive Cancer Center.
Aim is to enroll a total of 21 patients; patients will be treated up to 24 months or until disease progression.



